FDA Authorizes Modified Risk Tobacco Products
By A Mystery Man Writer
Last updated 15 Jun 2024

FDA authorized eight snus smokeless tobacco products to advertise with specific information about the lower health risks of using the snus products compared to smoking cigarettes.

Altria on X: Today, FDA authorized the marketing of the IQOS tobacco heating system as a modified risk tobacco product with a reduced exposure claim. / X

US FDA authorizes marketing of IQOS as a Modified Risk Tobacco Product

FDA Authorizes Tobacco Product But Emphasizes It's 'Not Safe

FDA Grants First-Ever Modified Risk Orders to General Snus

Tobacco Education Resource Library Site Search

Cuales son los productos con nicotina de riesgo reducido? » ARDT

Cuáles son los productos con nicotina de riesgo reducido

Why has the WHO FCTC failed to reduce adult smoking and its health impact? - Foundation for a Smoke-Free World

Tobacco harm reduction policy in spotlight

In a First, FDA Authorizes Marketing of Low-Nicotine Cigarette as Modified Risk Tobacco Product

Pulze: The science of heat not burn - Imperial Brands Science
:sharpen(level=0):output(format=jpeg)/up/dt/2019/11/FDA-grants-first-ever-modified-risk-orders-to-smokeless-tobacco-products.jpg)
DT News - International - FDA grants first modified risk orders to smokeless tobacco products
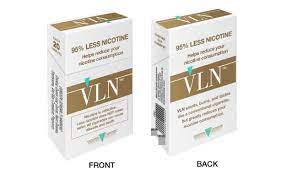
US FDA Gives MRTP to Low Nicotine Combustible Cigarette – Vapor Voice
Recommended for you
-
 Aluminum Snus can Snuffbox with lid grey 3 Layers Christmas Gifts snus can (Gray)15 Jun 2024
Aluminum Snus can Snuffbox with lid grey 3 Layers Christmas Gifts snus can (Gray)15 Jun 2024 -
 Snus Cans Aluminum Silver Color 3 Layers Snuffbox CNC Metal for Snus Packaging (Silver) : Health & Household15 Jun 2024
Snus Cans Aluminum Silver Color 3 Layers Snuffbox CNC Metal for Snus Packaging (Silver) : Health & Household15 Jun 2024 -
 The Snussie Can - Nano15 Jun 2024
The Snussie Can - Nano15 Jun 2024 -
 Tigersnus - Peppermint15 Jun 2024
Tigersnus - Peppermint15 Jun 2024 -
 Order Skoal Smooth Mint .58oz White Pouches ➝ Northerner US15 Jun 2024
Order Skoal Smooth Mint .58oz White Pouches ➝ Northerner US15 Jun 2024 -
 Icetool The Can for portion snus - Silver - Aluminum – Icetool snus accessories15 Jun 2024
Icetool The Can for portion snus - Silver - Aluminum – Icetool snus accessories15 Jun 2024 -
 Buy DUS Aluminum Original Size Can - Silver, SnusCentral15 Jun 2024
Buy DUS Aluminum Original Size Can - Silver, SnusCentral15 Jun 2024 -
 IceTool Slim Can Leatherface Red/Black - SnusPort15 Jun 2024
IceTool Slim Can Leatherface Red/Black - SnusPort15 Jun 2024 -
 Aluminum Snus can Snuffbox with lid gold color 3 Layers Christmas Gifts snus can (Gold)15 Jun 2024
Aluminum Snus can Snuffbox with lid gold color 3 Layers Christmas Gifts snus can (Gold)15 Jun 2024 -
 Icetool The Can for portion snus - Titan - Aluminum – Icetool snus accessories15 Jun 2024
Icetool The Can for portion snus - Titan - Aluminum – Icetool snus accessories15 Jun 2024
You may also like
-
 Glarks 116Pcs Seam Rippers Kit, 4Pcs Seam Rippers, 10Pcs Tailors Chalk, 1Pc Thread Snips, 100Pcs Multipurpose Sewing Clips Assorted Colors with a Cute Tin Box for Decoration, Craft and Sewing Tool15 Jun 2024
Glarks 116Pcs Seam Rippers Kit, 4Pcs Seam Rippers, 10Pcs Tailors Chalk, 1Pc Thread Snips, 100Pcs Multipurpose Sewing Clips Assorted Colors with a Cute Tin Box for Decoration, Craft and Sewing Tool15 Jun 2024 -
 Bullseye Neo-Lavender Shift Transparent Iridescent Thin 2mm 9015 Jun 2024
Bullseye Neo-Lavender Shift Transparent Iridescent Thin 2mm 9015 Jun 2024 -
 Dot Marker Positive Quotes Activity Book: dot marker coloring book for adults a book by Luckey Bob15 Jun 2024
Dot Marker Positive Quotes Activity Book: dot marker coloring book for adults a book by Luckey Bob15 Jun 2024 -
 How To Make Embellishments For Greeting Cards and Journals15 Jun 2024
How To Make Embellishments For Greeting Cards and Journals15 Jun 2024 -
 SOLDERING - TAPES - Copper Foil Tape - Grove Sales Ltd15 Jun 2024
SOLDERING - TAPES - Copper Foil Tape - Grove Sales Ltd15 Jun 2024 -
 Essential Elements by Candle-lite Scented Candles, Blood Orange & Teakwood Fragrance, One 14.75 oz. Three-Wick Aromatherapy Candle with 45 Hours of15 Jun 2024
Essential Elements by Candle-lite Scented Candles, Blood Orange & Teakwood Fragrance, One 14.75 oz. Three-Wick Aromatherapy Candle with 45 Hours of15 Jun 2024 -
 Styrofoam Balls 6-Inch, Each – King Stationary Inc15 Jun 2024
Styrofoam Balls 6-Inch, Each – King Stationary Inc15 Jun 2024 -
 Promotional Fine Point Wet Erase Markers - USA Made $0.5015 Jun 2024
Promotional Fine Point Wet Erase Markers - USA Made $0.5015 Jun 2024 -
 Gucci GG Supreme Monogram Black Canvas Leather Trim Web Strap Mini Tot – Queen Bee of Beverly Hills15 Jun 2024
Gucci GG Supreme Monogram Black Canvas Leather Trim Web Strap Mini Tot – Queen Bee of Beverly Hills15 Jun 2024 -
 Stichting Nidos Brother SE700 Sewing and Embroidery Machine – NEW NO RESERVE15 Jun 2024
Stichting Nidos Brother SE700 Sewing and Embroidery Machine – NEW NO RESERVE15 Jun 2024