Polysorbate 20 Degradation in Biopharmaceutical Formulations: Quantification of Free Fatty Acids, Characterization of Particulates, and Insights into the Degradation Mechanism
By A Mystery Man Writer
Last updated 01 Jun 2024


Polysorbate degradation in biotherapeutic formulations: Identification and discussion of current root causes

Hydrolytic polysorbate 20 degradation – Sensitive detection of free fatty acids in biopharmaceuticals via UPLC-QDa analytics with isolator column - ScienceDirect

Polysorbate degradation in biotherapeutic formulations: Identification and discussion of current root causes

Understanding Particle Formation: Solubility of Free Fatty Acids as Polysorbate 20 Degradation Byproducts in Therapeutic Monoclonal Antibody Formulations
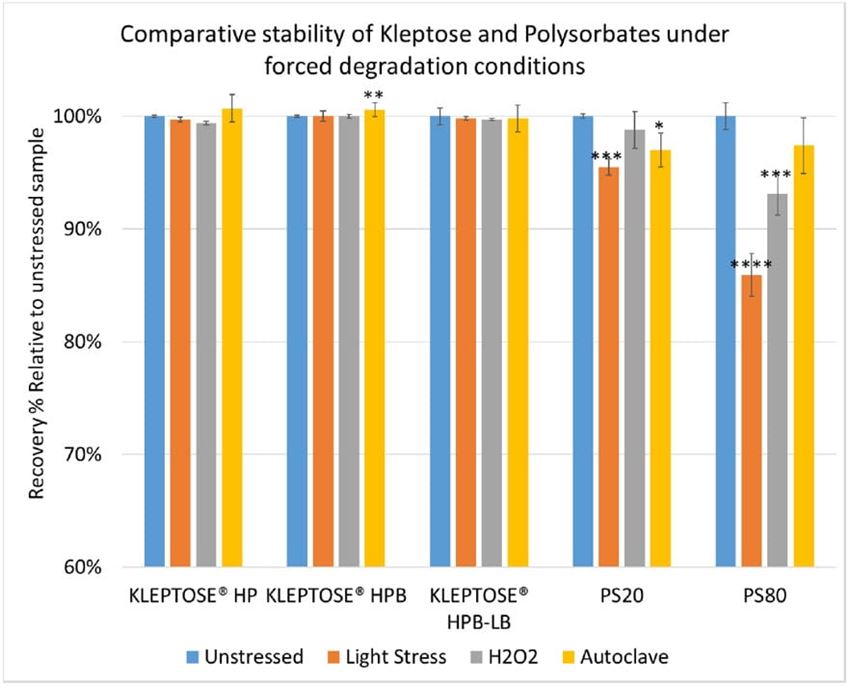
Polysorbates versus Hydroxypropyl Beta-Cyclodextrin: Comparative Study on Excipient Stability and Stabilization Benefits on Monoclonal Antibodies

Degradation of Polysorbate 20 by Sialate O-Acetylesterase in Monoclonal Antibody Formulations - Journal of Pharmaceutical Sciences

Improving Prediction of Free Fatty Acid Particle Formation in Biopharmaceutical Drug Products: Incorporating Ester Distribution during Polysorbate 20 Degradation

Controlled polysorbate 20 hydrolysis – A new approach to assess the impact of polysorbate 20 degradation on biopharmaceutical product quality in shortened time - ScienceDirect

Enzymatic degradation pattern of polysorbate 20 impacts interfacial properties of monoclonal antibody formulations - ScienceDirect

Improving Prediction of Free Fatty Acid Particle Formation in Biopharmaceutical Drug Products: Incorporating Ester Distribution during Polysorbate 20 Degradation

Trends on Analytical Characterization of Polysorbates and Their Degradation Products in Biopharmaceutical Formulations - Journal of Pharmaceutical Sciences
Recommended for you
-
 Polysorbate 20 (Solubilser) - 500g01 Jun 2024
Polysorbate 20 (Solubilser) - 500g01 Jun 2024 -
 Tween® 2001 Jun 2024
Tween® 2001 Jun 2024 -
 Soapeauty POLYSORBATE 20 T-MAZ 20, TWEEN 20 | 100% Pure Cosmetic Grade Solubilizer Surfactant & Emulsifier | (8 oz)01 Jun 2024
Soapeauty POLYSORBATE 20 T-MAZ 20, TWEEN 20 | 100% Pure Cosmetic Grade Solubilizer Surfactant & Emulsifier | (8 oz)01 Jun 2024 -
 Polysorbate 20 in Skin Care: Why Is It Bad for Your Skin? - Annmarie Gianni01 Jun 2024
Polysorbate 20 in Skin Care: Why Is It Bad for Your Skin? - Annmarie Gianni01 Jun 2024 -
Tween® 20, 250 g, CAS No. 9005-64-5, Detergents, Biochemistry, Life Science01 Jun 2024
-
 Polysorbate 20 per lb. Candles and Supplies.net01 Jun 2024
Polysorbate 20 per lb. Candles and Supplies.net01 Jun 2024 -
 Quantitative Analysis of Polysorbate 20/80 in Protein-Based01 Jun 2024
Quantitative Analysis of Polysorbate 20/80 in Protein-Based01 Jun 2024 -
 POLYSORBATE 20 NF - PCCA01 Jun 2024
POLYSORBATE 20 NF - PCCA01 Jun 2024 -
 Hydrolysis of Polysorbate 20 and 80 by a Range of Carboxylester01 Jun 2024
Hydrolysis of Polysorbate 20 and 80 by a Range of Carboxylester01 Jun 2024 -
 Understanding the Practical Differences Between Polysorbate 20 and01 Jun 2024
Understanding the Practical Differences Between Polysorbate 20 and01 Jun 2024
You may also like
-
 ONine Microfiber Couch Repair Kit, 55x12 inch Adhesive01 Jun 2024
ONine Microfiber Couch Repair Kit, 55x12 inch Adhesive01 Jun 2024 -
Benefit Cosmetics Superstar Wardrobe Mini Makeup Set - SAVE 55%01 Jun 2024
-
 Building a Texaco Doodlebug with a Quad-turbo V16 – Engine Swap Depot01 Jun 2024
Building a Texaco Doodlebug with a Quad-turbo V16 – Engine Swap Depot01 Jun 2024 -
 Personalized leather UNO card case Custom leather UNO card holder - Shop Sunbrilo Other - Pinkoi01 Jun 2024
Personalized leather UNO card case Custom leather UNO card holder - Shop Sunbrilo Other - Pinkoi01 Jun 2024 -
 AB Diamond Painting - Square Drill - Purple Waterfall(45*75cm)-106174401 Jun 2024
AB Diamond Painting - Square Drill - Purple Waterfall(45*75cm)-106174401 Jun 2024 -
 JennaKate Dry Erase Chores Reward Chart Grey01 Jun 2024
JennaKate Dry Erase Chores Reward Chart Grey01 Jun 2024 -
 d8ce80e9914026a3a7f23c77667e8963–gothic-chokers-gothic-jewelry – Words By Janeen G.01 Jun 2024
d8ce80e9914026a3a7f23c77667e8963–gothic-chokers-gothic-jewelry – Words By Janeen G.01 Jun 2024 -
 Gum arabic, what is it used for? Discover how this gum helps sugarcrafters01 Jun 2024
Gum arabic, what is it used for? Discover how this gum helps sugarcrafters01 Jun 2024 -
 Expressions Journal Kit01 Jun 2024
Expressions Journal Kit01 Jun 2024 -
 Hot Fix Hair Gems – Insert Name Here01 Jun 2024
Hot Fix Hair Gems – Insert Name Here01 Jun 2024
