FDA Grants Import Discretion of Bracco's Iodinated Contrast Medium
By A Mystery Man Writer
Last updated 02 Jun 2024
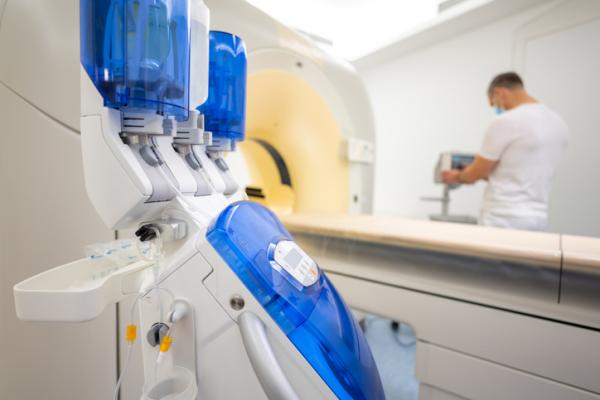
Bracco Diagnostics Inc. has announced that the U.S. Food and Drug Administration (FDA) granted import discretion of Iomeron (iomeprol injection) into the U.S. to address the ongoing iodinated contrast media shortage. The product addresses the need for the most advanced diagnostic imaging standards and will be temporarily available in the U.S. market starting at the end of August, 2022.
Kodak Receives FDA Clearance for New DR System

Ultravist (Iopromide) Paves New Path in Breast Cancer Detection - Xtalks
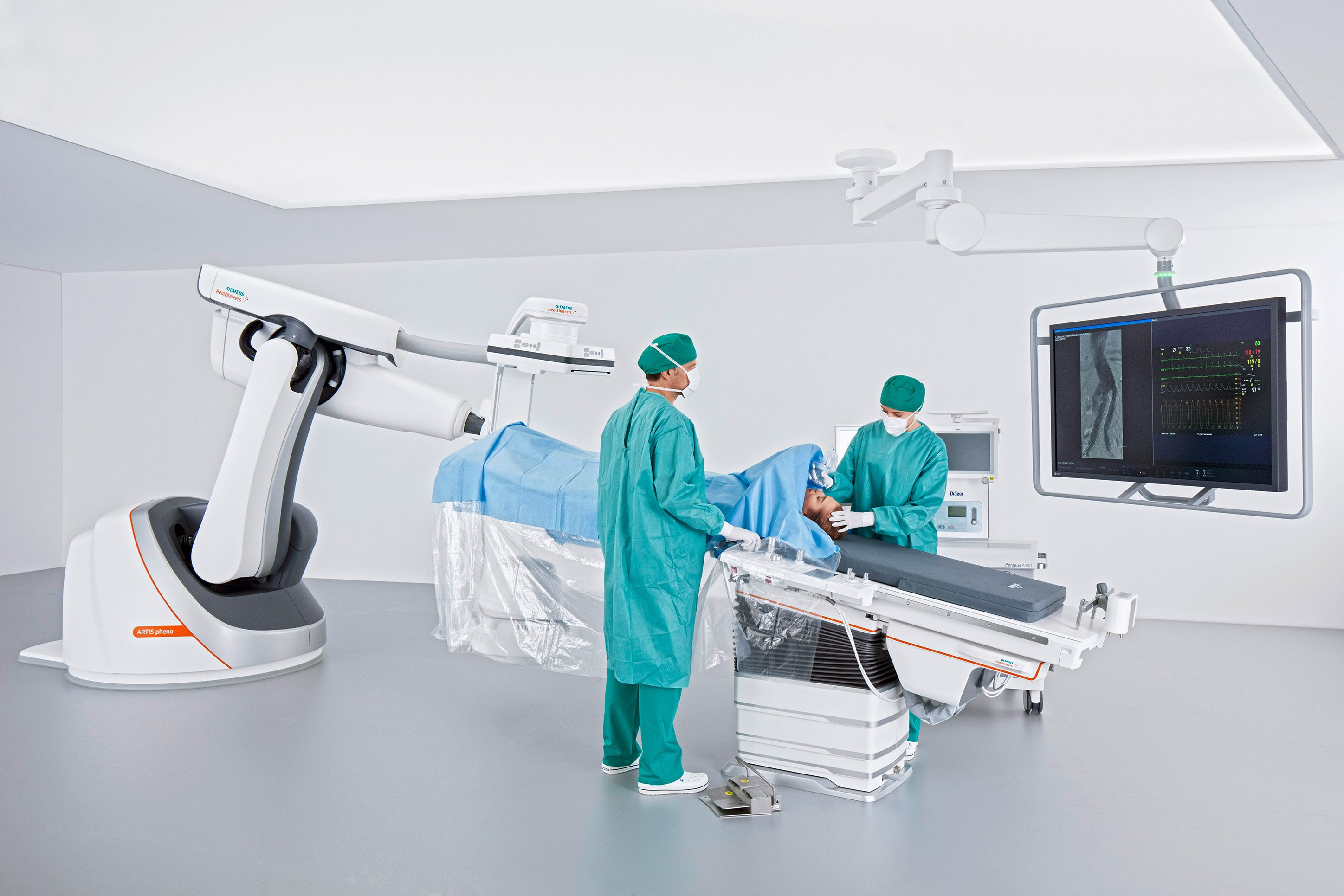
FDA Clears Artis Pheno Angiography System from Siemens Healthineers

Short-, Mid-, and Long-term Strategies to Manage the Shortage of Iohexol
Guerbet Announces ACR Committee on Drugs and Contrast Media Classifies Elucirem (Gadopiclenol) Injection a Group II Agent

Contrast Media Imaging Technology News
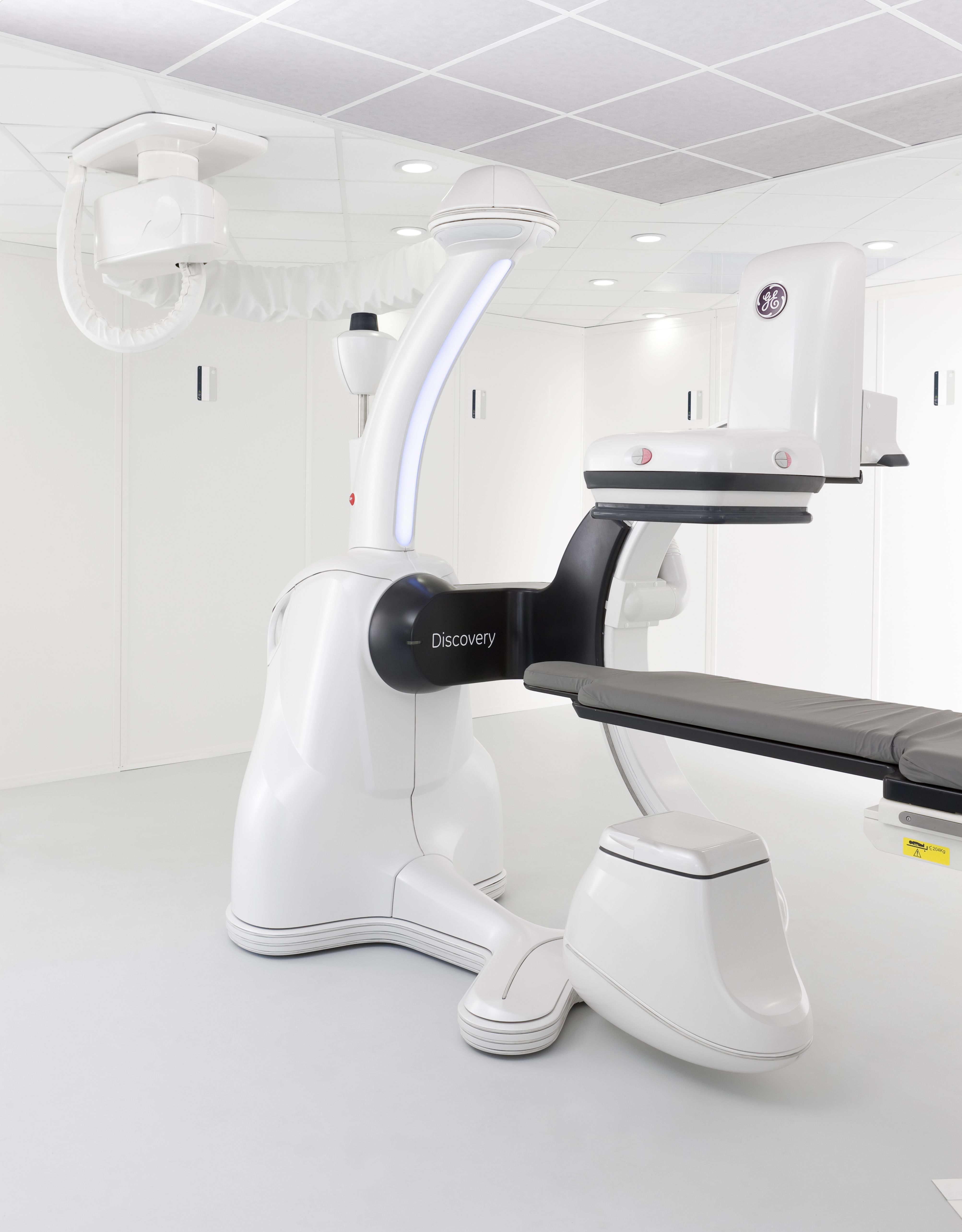
FDA Clears GE Healthcare's New Class of Angiography Systems

EHR Interventions for Contrast Media Shortage Impact CT Utilization
Siemens Healthineers Announces FDA Clearance of ARTIS icono ceiling Angiography System
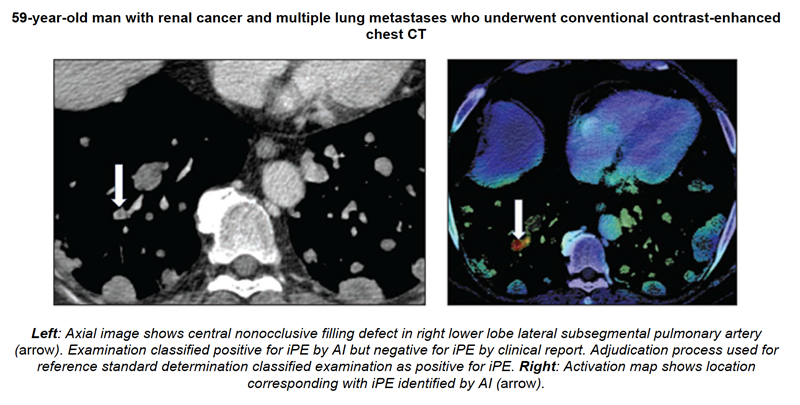
articles • APPLIED RADIOLOGY
Shimadzu Announces First U.S. Trinias Digital Angiography System Installation

Bracco wins EU approval for MRI contrast agent - Medical Device Network
ACIST Features Contrast Injectors, Software
First Nationwide Use of Bracco's VUEWAY (gadopiclenol) Solution for Injection for MRI
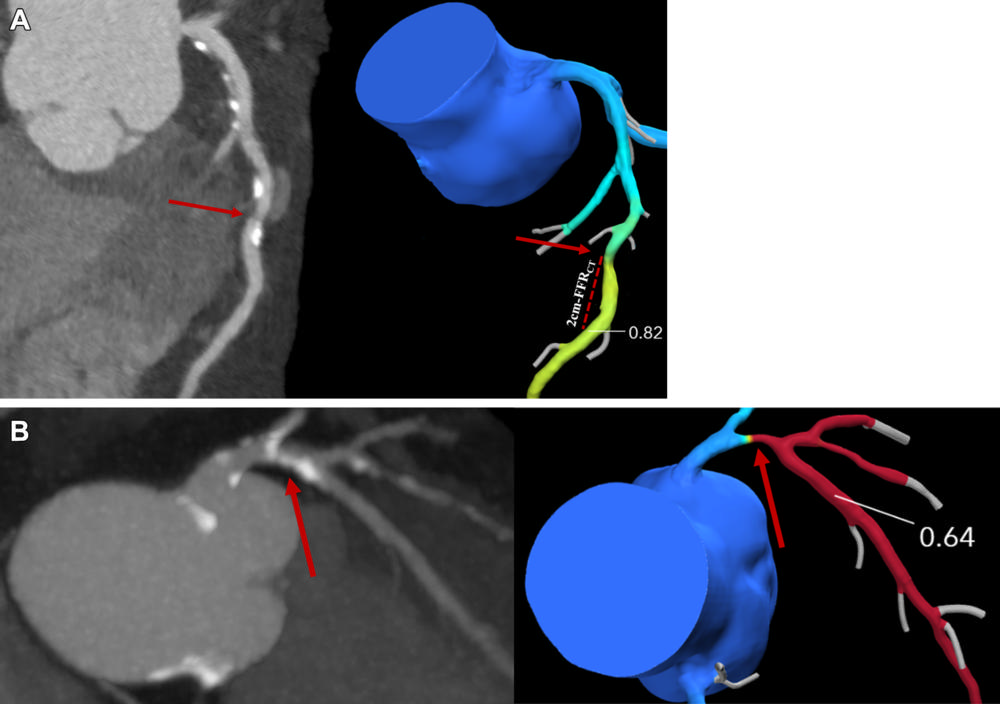
articles • APPLIED RADIOLOGY
Recommended for you
-
 Games Workshop Citadel Pot de Peinture - Technical Contrast Medium (24ml)02 Jun 2024
Games Workshop Citadel Pot de Peinture - Technical Contrast Medium (24ml)02 Jun 2024 -
 About contrast agents - Mysurgeryabroad - Medicover Hospital - Hospital abroad, affordable surgery in Budapest, Hungary02 Jun 2024
About contrast agents - Mysurgeryabroad - Medicover Hospital - Hospital abroad, affordable surgery in Budapest, Hungary02 Jun 2024 -
 Medical Imaging 100ml CT Contrast Medium Syringes for Seacrown Zenith-C10 Angiographic Injectors – Disposable Syringes Suppliers for Medrad Liebel Flarsheim Nemoto Medtron CT MRI ANGIO CATH LAB Contrast Media Injectors02 Jun 2024
Medical Imaging 100ml CT Contrast Medium Syringes for Seacrown Zenith-C10 Angiographic Injectors – Disposable Syringes Suppliers for Medrad Liebel Flarsheim Nemoto Medtron CT MRI ANGIO CATH LAB Contrast Media Injectors02 Jun 2024 -
 Contrast Media Genetek Lifesciences02 Jun 2024
Contrast Media Genetek Lifesciences02 Jun 2024 -
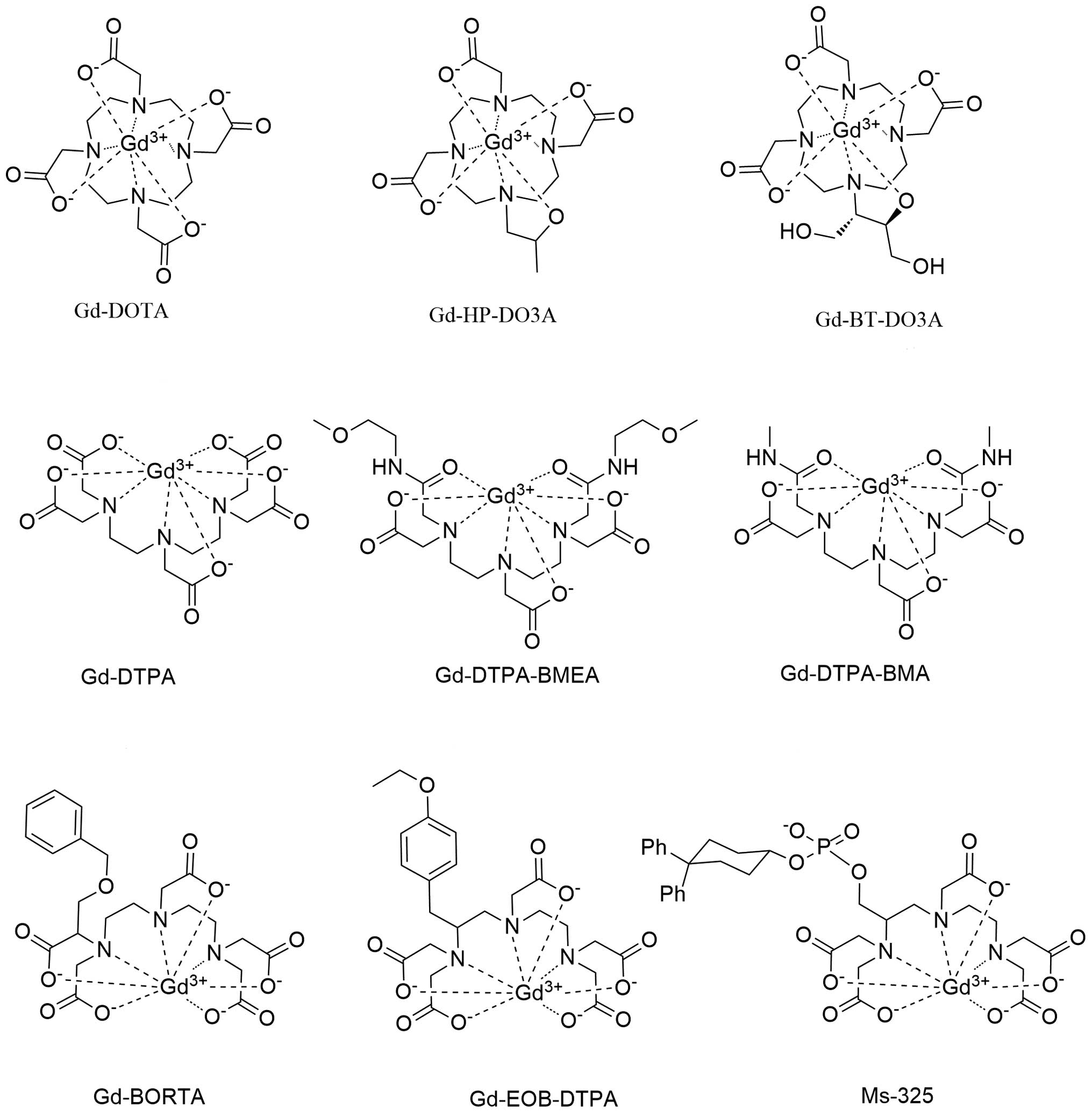 MRI contrast agents: Classification and application (Review)02 Jun 2024
MRI contrast agents: Classification and application (Review)02 Jun 2024 -
Nontraumatic intravasation of myelographic contrast medium02 Jun 2024
-
 Electroanatomical Navigation to Minimize Contrast Medium or X-Rays During Stenting: Insights From an Experimental Model - ScienceDirect02 Jun 2024
Electroanatomical Navigation to Minimize Contrast Medium or X-Rays During Stenting: Insights From an Experimental Model - ScienceDirect02 Jun 2024 -
 Technical contrast medium review02 Jun 2024
Technical contrast medium review02 Jun 2024 -
 Contrast medium hi-res stock photography and images - Alamy02 Jun 2024
Contrast medium hi-res stock photography and images - Alamy02 Jun 2024 -
Contrast medium, a sample of the element Gadolinium in the02 Jun 2024
You may also like
-
 Croc Jibbitz Letters02 Jun 2024
Croc Jibbitz Letters02 Jun 2024 -
 Breville Mini Smart Oven with Element IQ02 Jun 2024
Breville Mini Smart Oven with Element IQ02 Jun 2024 -
 Sanrio Hello Kitty Kuromi My Melody Cinnamoroll Cute Pencil Bag02 Jun 2024
Sanrio Hello Kitty Kuromi My Melody Cinnamoroll Cute Pencil Bag02 Jun 2024 -
 Reviews for Niagara 20 oz. Heavy Spray Starch02 Jun 2024
Reviews for Niagara 20 oz. Heavy Spray Starch02 Jun 2024 -
 Jumbo Gold Foil 30 sq ft. Gift Wrapping Paper Rolls - Sold individually02 Jun 2024
Jumbo Gold Foil 30 sq ft. Gift Wrapping Paper Rolls - Sold individually02 Jun 2024 -
 Apple Cider Punch (Super Easy Fall Punch Recipe) - Pizzazzerie02 Jun 2024
Apple Cider Punch (Super Easy Fall Punch Recipe) - Pizzazzerie02 Jun 2024 -
 Red and Green Mini Christmas Tree Beads02 Jun 2024
Red and Green Mini Christmas Tree Beads02 Jun 2024 -
 Greeting Card Paper - 5 X 7 | 80lb | White - (Envelopes Included)02 Jun 2024
Greeting Card Paper - 5 X 7 | 80lb | White - (Envelopes Included)02 Jun 2024 -
 Finished Diamond Painting,Teddy Bears in the Garden,Diamond Art,Handmade Painting,3d Painting,Diamond Dots,Handmade Artwork,Country Wall Art02 Jun 2024
Finished Diamond Painting,Teddy Bears in the Garden,Diamond Art,Handmade Painting,3d Painting,Diamond Dots,Handmade Artwork,Country Wall Art02 Jun 2024 -
 6/12pcs Fix Zipper Slider Zipper Head Universal Kit Replacement Instant Repair Zipper Convenient Useful Free Shipping&Wholesales - Price history & Review, AliExpress Seller - 525Home Store02 Jun 2024
6/12pcs Fix Zipper Slider Zipper Head Universal Kit Replacement Instant Repair Zipper Convenient Useful Free Shipping&Wholesales - Price history & Review, AliExpress Seller - 525Home Store02 Jun 2024