Polymorph screening in pharmaceutical development - European Pharmaceutical Review
By A Mystery Man Writer
Last updated 18 Jun 2024
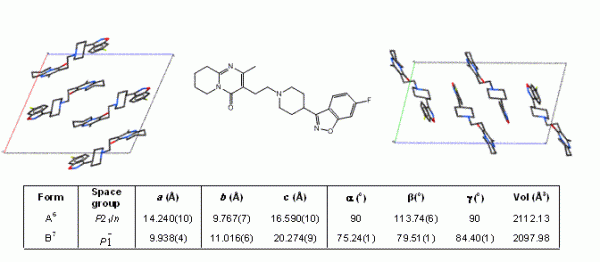
The majority of active pharmaceutical ingredients (APIs) are produced by crystallisation and so the phenomenon of polymorphism, whereby an organic molecule can adopt more than one crystalline form (Figure 1), is of considerable importance when trying to achieve consistent product quality during the manufacture of pharmaceutical solids and solid dosage forms. Although morphology and particle size-distribution are important solid-state characteristics, the uncontrolled occurrence of multiple physical forms (polymorphs, solvates, salts, co-crystals or amorphous) of an API can have significant effects on the performance of the material during processing, manufacture, storage and administration. For example, the solubility difference between some polymorphs has been shown to be over four times that of the least soluble form1 and can vary by significantly more for amorphous forms2.
Polymorph screening in pharmaceutical development - European

Weak Interactions Cause Packing Polymorphism in Pharmaceutical Two

High-throughput electronic biology: mining information for drug

Solid form changes during drug development: good, bad, and ugly

WO2021032959A1 - Crystalline forms of pyrimidino diazepine derivative - Google Patents

Current applications of Multivariate Curve Resolution- Alternating
Polymorph screening in pharmaceutical development - European

A practical guide to pharmaceutical polymorph screening
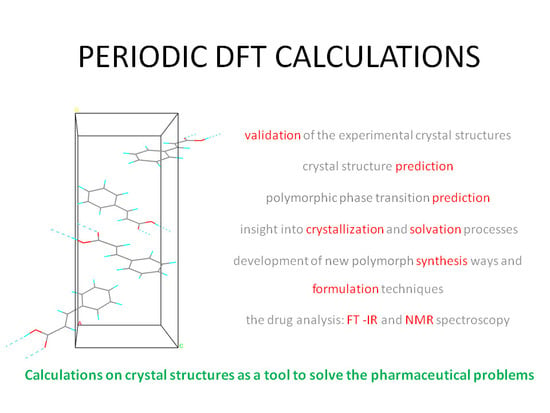
Pharmaceutics, Free Full-Text
Crystals, Free Full-Text
Recommended for you
-
 Polymorph · Magic 2010 (M10) #67 · Scryfall Magic The Gathering Search18 Jun 2024
Polymorph · Magic 2010 (M10) #67 · Scryfall Magic The Gathering Search18 Jun 2024 -
![Mordenkainen's Polymorph [Dungeons & Dragons: Adventures in the Forgotten Realms]](https://thecleverkobold.com/cdn/shop/products/581ffa37-2a6a-5fd3-bf44-77e84ef66fbd_21e798d4-820a-4371-817a-a24d48587ea7.jpg?v=1629303880) Mordenkainen's Polymorph [Dungeons & Dragons: Adventures in the Forgotten Realms]18 Jun 2024
Mordenkainen's Polymorph [Dungeons & Dragons: Adventures in the Forgotten Realms]18 Jun 2024 -
![True Polymorph [Dungeons & Dragons: Adventures in the Forgotten Realms]](http://www.dungeonsandjavas.com/cdn/shop/products/3b60b94d-7a9c-5f32-99cd-996a44a53875.jpg?v=1682595491) True Polymorph [Dungeons & Dragons: Adventures in the Forgotten Realms]18 Jun 2024
True Polymorph [Dungeons & Dragons: Adventures in the Forgotten Realms]18 Jun 2024 -
 Major Brushes Polymorph (Moreform) 1000g18 Jun 2024
Major Brushes Polymorph (Moreform) 1000g18 Jun 2024 -
 Polymorphism in Pharmaceutical Drug Development18 Jun 2024
Polymorphism in Pharmaceutical Drug Development18 Jun 2024 -
 Stormsure Polymorph Hand-Mouldable Plastic Granules 500g18 Jun 2024
Stormsure Polymorph Hand-Mouldable Plastic Granules 500g18 Jun 2024 -
 Polymorph Thermoformable Plastic - 1000g - RobotShop18 Jun 2024
Polymorph Thermoformable Plastic - 1000g - RobotShop18 Jun 2024 -
 Polymorph Build Warcraft Rumble18 Jun 2024
Polymorph Build Warcraft Rumble18 Jun 2024 -
 Polymorph (1996) - IMDb18 Jun 2024
Polymorph (1996) - IMDb18 Jun 2024 -
 ESUN Polymorph Hand Moldable Plastic (250g Bottle)18 Jun 2024
ESUN Polymorph Hand Moldable Plastic (250g Bottle)18 Jun 2024
You may also like
-
 Key Chain Kits – Turners Warehouse18 Jun 2024
Key Chain Kits – Turners Warehouse18 Jun 2024 -
 KitchenAid All Purpose Shears with Protective Sheath18 Jun 2024
KitchenAid All Purpose Shears with Protective Sheath18 Jun 2024 -
 Sterling Silver Graduating Beaded Necklace18 Jun 2024
Sterling Silver Graduating Beaded Necklace18 Jun 2024 -
 How much Wacom nibs lasts? And how to make your graphics tablet18 Jun 2024
How much Wacom nibs lasts? And how to make your graphics tablet18 Jun 2024 -
 E-6000 Clear Industrial Adhesive Medium Viscosity Glue 3.7 oz. 230022 by E6000 : : Tools & Home Improvement18 Jun 2024
E-6000 Clear Industrial Adhesive Medium Viscosity Glue 3.7 oz. 230022 by E6000 : : Tools & Home Improvement18 Jun 2024 -
clip fufu 3D Models to Print - yeggi18 Jun 2024
-
 STOBOK 12 Pcs Watercolor Painting Brushes Pen Watercolor Markers for Adults Kits Water Coloring Brush Paint Brush Suit for Painting Pens Watercolor18 Jun 2024
STOBOK 12 Pcs Watercolor Painting Brushes Pen Watercolor Markers for Adults Kits Water Coloring Brush Paint Brush Suit for Painting Pens Watercolor18 Jun 2024 -
 Caron Simply Soft Brites Yarn - Berry Blue18 Jun 2024
Caron Simply Soft Brites Yarn - Berry Blue18 Jun 2024 -
 Pazaka Vinyl Letter Stickers Mailbox Numbers 10 Sheets, Self-Adhesive Letter for Mailbox Address Home Business Kitchen Containers, Cup, Jar (2 Inch, Black) : Arts, Crafts & Sewing18 Jun 2024
Pazaka Vinyl Letter Stickers Mailbox Numbers 10 Sheets, Self-Adhesive Letter for Mailbox Address Home Business Kitchen Containers, Cup, Jar (2 Inch, Black) : Arts, Crafts & Sewing18 Jun 2024 -
 Blue jay Pack of 10 Rangoli Powder Price in India - Buy Blue jay18 Jun 2024
Blue jay Pack of 10 Rangoli Powder Price in India - Buy Blue jay18 Jun 2024
