Polysorbate 20 degradation in biotherapeutic formulations and its
By A Mystery Man Writer
Last updated 07 Jun 2024
Enzyme-induced hydrolysis of polysorbate 20 and formation of subvisible particles was evaluated using relevant analytical techniques to understand the impact of

PDF) Identification of the specific causes of polysorbate 20 degradation in monoclonal antibody formulations containing multiple lipases
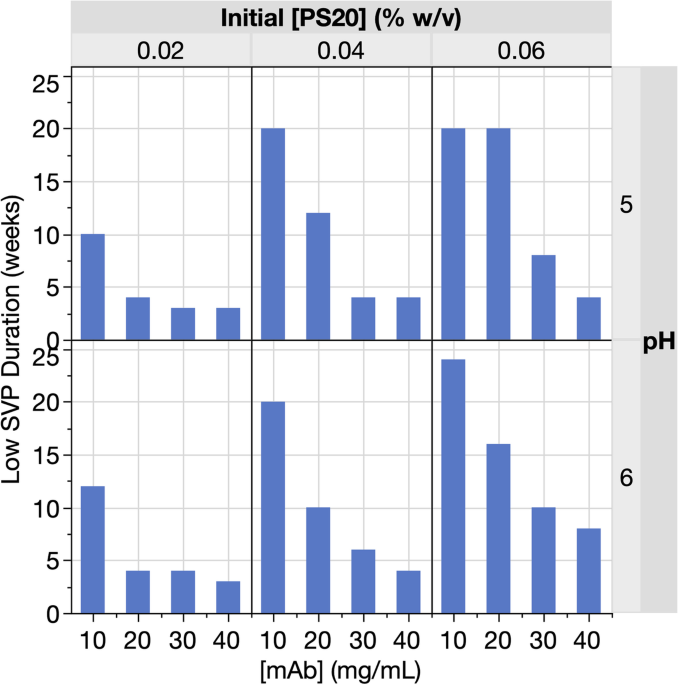
Formulation mitigations for particle formation induced by enzymatic hydrolysis of polysorbate 20 in protein-based drug products: insights from a full-factorial longitudinal study, AAPS Open

Quantitative Analysis of Polysorbate 20/80 in Protein-Based Biopharmaceuticals Using A One-Pot RPLC-MS Based Platform Method

Polysorbate, the Good, the Bad and the Ugly American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology

Degradation of polysorbates 20 and 80 catalysed by histidine chloride buffer - ScienceDirect

Profiling Active Enzymes for Polysorbate Degradation in Biotherapeutics by Activity-Based Protein Profiling
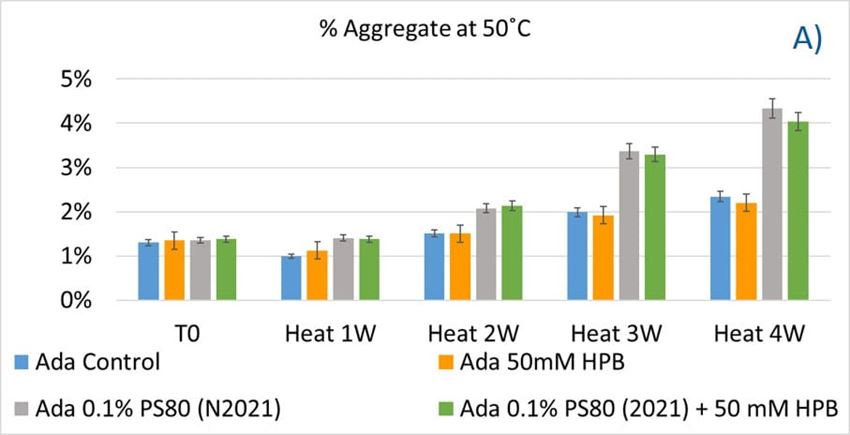
Polysorbates versus Hydroxypropyl Beta-Cyclodextrin: Comparative Study on Excipient Stability and Stabilization Benefits on Monoclonal Antibodies

Polysorbate degradation in biotherapeutic formulations: Identification and discussion of current root causes - ScienceDirect
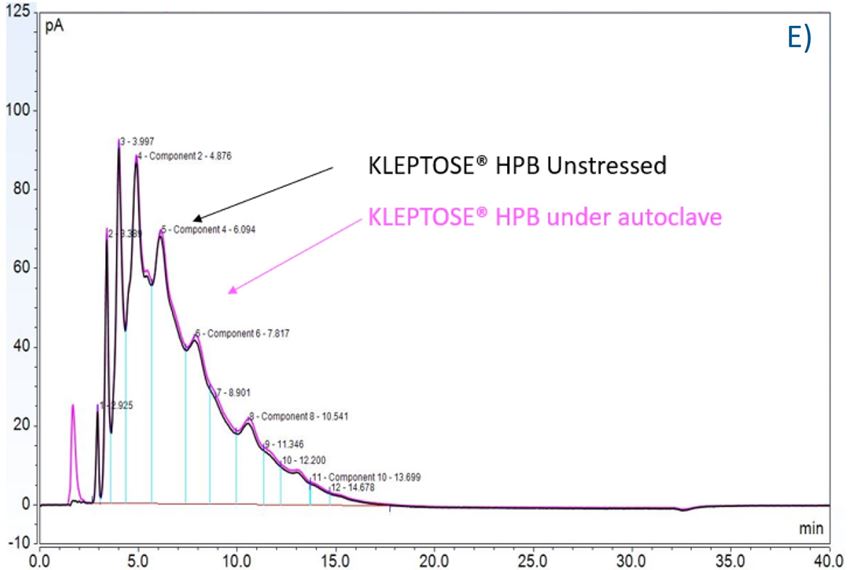
Polysorbates versus Hydroxypropyl Beta-Cyclodextrin: Comparative Study on Excipient Stability and Stabilization Benefits on Monoclonal Antibodies
View of ANALYSIS OF POLYSORBATE 80 SOLUTION STABILITY UNDER STRESS CONDITIONS TO ENSURE ITS QUALITY AS A BIOPHARMACEUTICAL EXCIPIENT

Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: Structure and degradation pathways - Kerwin - 2008 - Journal of Pharmaceutical Sciences - Wiley Online Library
Recommended for you
-
 Polysorbate 20 T-maz 20 Tween 20 Solubilizer Surfactant & Emulsifier 100% Pure 2 oz07 Jun 2024
Polysorbate 20 T-maz 20 Tween 20 Solubilizer Surfactant & Emulsifier 100% Pure 2 oz07 Jun 2024 -
 Polysorbate 2007 Jun 2024
Polysorbate 2007 Jun 2024 -
 Polysorbate 20 13207 Jun 2024
Polysorbate 20 13207 Jun 2024 -
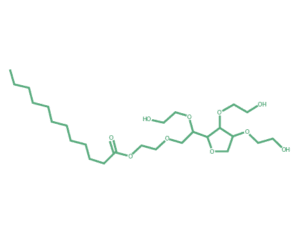 Polysorbate 20, CAS: 9005-64-5, Request a Quote07 Jun 2024
Polysorbate 20, CAS: 9005-64-5, Request a Quote07 Jun 2024 -
 Tween-20, CAS 9005-64-507 Jun 2024
Tween-20, CAS 9005-64-507 Jun 2024 -
 Polysorbate-20 (Tween 20) - Purenso Select07 Jun 2024
Polysorbate-20 (Tween 20) - Purenso Select07 Jun 2024 -
![Polysorbate 20 [CAS_9005-64-5] NF/FCC Liquid (485 Lb Drum](https://www.wintersunchemical.com/cdn/shop/products/16-117-1.jpg?v=1666132893) Polysorbate 20 [CAS_9005-64-5] NF/FCC Liquid (485 Lb Drum07 Jun 2024
Polysorbate 20 [CAS_9005-64-5] NF/FCC Liquid (485 Lb Drum07 Jun 2024 -
 Polysorbate 20 in Skin Care: Why Is It Bad for Your Skin07 Jun 2024
Polysorbate 20 in Skin Care: Why Is It Bad for Your Skin07 Jun 2024 -
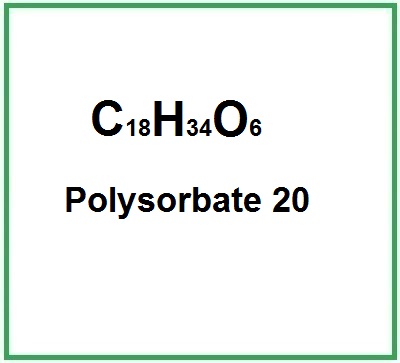 Polysorbate 2007 Jun 2024
Polysorbate 2007 Jun 2024 -
 Polysorbate, the Good, the Bad and the Ugly07 Jun 2024
Polysorbate, the Good, the Bad and the Ugly07 Jun 2024
You may also like
-
 112 Sheets Origami Foil Paper Art Cardstock Paper Metallic Colors Craft Mirror Paper Bright Board Sheets Folding Paper Scrapbook Paper, DIY Card, Arts07 Jun 2024
112 Sheets Origami Foil Paper Art Cardstock Paper Metallic Colors Craft Mirror Paper Bright Board Sheets Folding Paper Scrapbook Paper, DIY Card, Arts07 Jun 2024 -
 Daler-Rowney Marker Pad: 50 Pages, 70 gsm, Paperback – Perfect Paper Company07 Jun 2024
Daler-Rowney Marker Pad: 50 Pages, 70 gsm, Paperback – Perfect Paper Company07 Jun 2024 -
 FOLLOWIN Gloss Black Rim Touch Up Paint for Cars, Black Wheel Paint Repair Kit, Automotive Rim Scratch Repair, Touch up Paint Kit with Brush, Repair07 Jun 2024
FOLLOWIN Gloss Black Rim Touch Up Paint for Cars, Black Wheel Paint Repair Kit, Automotive Rim Scratch Repair, Touch up Paint Kit with Brush, Repair07 Jun 2024 -
 ECHSRT Large Pencil Case, Durable Pen Pouch with Big Capacity, Minimalist Portable Stationery Bag with Handle for Office Organizer Aesthetic Pencil07 Jun 2024
ECHSRT Large Pencil Case, Durable Pen Pouch with Big Capacity, Minimalist Portable Stationery Bag with Handle for Office Organizer Aesthetic Pencil07 Jun 2024 -
 Grab & Go Craft Kits for Adults and Teens - Waterville Creates07 Jun 2024
Grab & Go Craft Kits for Adults and Teens - Waterville Creates07 Jun 2024 -
 Professional Sketch Drawing Pencil Set Hb 2b 6h 4h 2h 3b 4b - Temu07 Jun 2024
Professional Sketch Drawing Pencil Set Hb 2b 6h 4h 2h 3b 4b - Temu07 Jun 2024 -
 Crochet Knitting Pattern Books Baby Vintage 1955 Quick Knit Easy to Make07 Jun 2024
Crochet Knitting Pattern Books Baby Vintage 1955 Quick Knit Easy to Make07 Jun 2024 -
 BEAUTY OUT OF CHAOS (charcoal drawing tutorial)07 Jun 2024
BEAUTY OUT OF CHAOS (charcoal drawing tutorial)07 Jun 2024 -
 Wholesale 5.9*10.6CM White Color Diamond Painting Tools Plastic Blank Rhinestone Trays for Nail Art DIY Crafts Painting From m.07 Jun 2024
Wholesale 5.9*10.6CM White Color Diamond Painting Tools Plastic Blank Rhinestone Trays for Nail Art DIY Crafts Painting From m.07 Jun 2024 -
 Winsor & Newton Artists' Oil Hog Paint Brush, Long Handle Size 2, Flat07 Jun 2024
Winsor & Newton Artists' Oil Hog Paint Brush, Long Handle Size 2, Flat07 Jun 2024